Defibrillators Information
Defibrillators are life-saving medical devices that are used to restore normal heart rhythm in individuals experiencing cardiac arrest. These devices are an essential piece of equipment in hospitals, ambulance services, and other healthcare settings, as well as in public places such as airports, schools, and office buildings.
Each year, thousands of lives are saved through the use of defibrillators. When cardiac arrest occurs, the person must be treated within minutes because the brain is deprived of oxygenated blood until the heart can begin pumping again. Quick access to a defibrillator can allow non-medical personnel to begin treating a patient, buying the patient crucial time until medical personnel arrives.

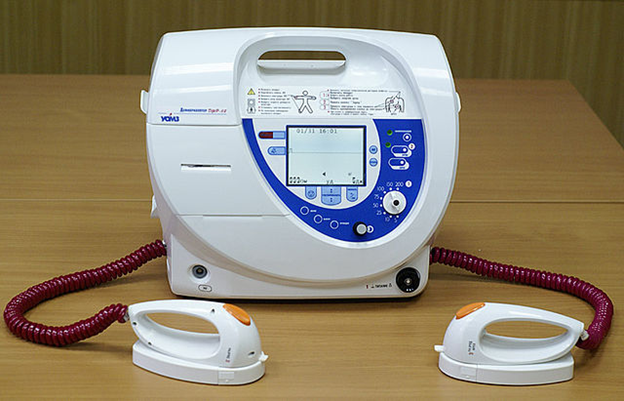
Figure 1: A defibrillator. Source: Pixabay
Theory of Application
Defibrillators are medical devices that are used to restore normal heart rhythm in individuals experiencing cardiac arrest. Cardiac arrest is a medical emergency that occurs when the heart stops beating effectively, and it can be caused by a variety of factors such as a heart attack, an abnormal heart rhythm, or other underlying health conditions.
Defibrillators work by delivering an electric shock to the heart in order to restore normal heart rhythm. This electric shock is delivered through a pair of paddles or pads that are placed on the chest of the patient. When the shock is delivered, it disrupts the abnormal heart rhythm and allows the heart to return to its normal rhythm.

Figure 2: The defibrillator will first assess the heart rhythm of the patient and determine whether or not a shock is needed. Source: Pixabay
There are two main types of defibrillators: manual defibrillators and automated external defibrillators (AEDs). Manual defibrillators are operated by trained medical professionals, who use the paddles to deliver the electric shock and manually adjust the intensity and duration of the shock based on the patient's condition. AEDs, on the other hand, are automated devices that use voice prompts and visual instructions to guide the user through the defibrillation process.
In both cases, the defibrillator will first assess the heart rhythm of the patient and determine whether or not a shock is needed. If a shock is needed, the defibrillator will deliver the shock and then reassess the patient's heart rhythm to see if the shock was successful in restoring normal rhythm. If the shock was not successful, the defibrillator may deliver additional shocks as needed.
Specifications
When comparing defibrillators some key specifications to review include:
Operating Mode
Fully automated and manual defibrillators offer different levels of control and require different levels of training. Manual defibrillators should only be used in medical settings by trained medical professionals. Fully automated defibrillators are often designed to be used by bystanders with no prior training.
Wave Form
Biphasic waveforms are the most popular and effective form of defibrillation although monophase waveforms are often available as well.
Size and weight
Defibrillators may come in different sizes and weights, depending on the intended use. For example, portable AEDs may be smaller and lighter than manual defibrillators.
Delivered Energy
Defibrillators often have either continuously adjustable power settings or may offer simply a high and low energy level setting. Some defibrillators are designed for use on either adults or children, often selectable with a button.
Water resistance
Some defibrillators may be designed to be water-resistant, which can be important in certain environments.
Shock Sequence
The sequence of the shocks can vary. Constant and escalating modes are common sequences.
Charging Time
The time required for the defibrillator to charge before delivering a shock is incredibly important. Understanding the typical and maximum times will determine how quickly a patient can be shocked.
Types
There are several different types of defibrillators that are used in various medical settings and public places. These devices are all designed to deliver an electric shock to the heart in order to restore normal heart rhythm in individuals experiencing cardiac arrest. Here are some of the different types of defibrillators:
Manual Defibrillators
These are the traditional type of defibrillators and they require a trained medical professional to operate them. Manual defibrillators consist of a device that delivers the electric shock, as well as paddles that are placed on the chest of the patient. The medical professional uses the paddles to deliver the shock and manually adjusts the intensity and duration of the shock based on the patient's condition.
Figure 3: Manual defibrillator. Source: Pixabay
AEDs
AEDs are designed for use by laypeople, and they are typically found in public places such as airports, schools, and office buildings. AEDs are automated devices that use voice prompts and visual instructions to guide the user through the defibrillation process. They are typically smaller and more portable than manual defibrillators and are easy to use even by individuals with no medical training.
Figure 4: An AED. Source: Pixabay
Implantable Cardioverter Defibrillators (ICDs)
ICDs are small devices that are implanted in the chest or abdomen of patients who are at high risk for sudden cardiac arrest. These devices are connected to the heart and can monitor the heart's rhythm and deliver a shock if necessary to restore normal rhythm. ICDs are typically used in patients who have experienced cardiac arrest in the past or who have certain heart conditions that put them at risk for sudden cardiac arrest.
Wearable defibrillators
Wearable defibrillators are small, portable devices that can be worn on the body and are designed to be used in the event of a cardiac arrest. These devices can be worn as a vest or as a patch and are equipped with sensors that monitor the heart's rhythm. If the device detects an abnormal rhythm, it can deliver a shock to restore normal rhythm. Wearable defibrillators are typically used in patients who are at high risk for sudden cardiac arrest but who may not be candidates for an ICD.
Features
While AEDs often limit the features available to the person operating the defibrillator, manual defibrillators give much more freedom. Common features include:
- Energy selection to vary the amount of energy delivered in the shock
- Disarm to allow the machine to discharge energy internally
- Sync to match the shock to the patient’s ECG rhythm
Construction
Figure 5: First responder kit. Source: Pixabay
The main parts of a defibrillator are the control box casing, microelectronics, batteries, and electrodes. All of these components must be made with biocompatible materials.
Control Box Casing
Typically made from hard polystyrene plastic or a lightweight metal alloy, the casing design and choice of materials depend on where and how the defibrillator is intended to be used. The control box casing must protect the defibrillator in transit and keep it safe and functional until it is needed.
Microelectronics
Made from modified silicon semiconductors, the microelectronics must be able to provide energy to the electrodes in a controlled and reliable manner. The capacitors used in defibrillators often hold 7 kV or more of electricity and must be able to deliver between 30 and 400 joules of energy.
Batteries
Battery construction varies based on the defibrillator application, but common chemistries include:
- Alkaline
- Lead acid
- Nickel-cadmium
- Zinc
- Lithium
- Sulfur dioxide
- Manganese dioxide
Electrodes
Required to deliver the shock to the patient, electrodes are typically made from performance materials like titanium. Silicone rubber is often used to insulate the electrodes. Large paddles allow for current to flow to the heart with less resistance.
Applications
Figure 6: An AED. Source: Pixabay
There are several types of defibrillators, including manual defibrillators, AEDs, and implanted defibrillators. Each type has its own specific applications.
Here are some common applications for manual and AED defibrillators:
Emergency Care
Manual defibrillators are often used in hospitals and other healthcare settings to treat patients with cardiac arrhythmias. Emergency medical services (EMS) personnel often carry manual defibrillators to use in the field. AEDs may also be carried by first responders such as police officers and firefighters.
Sports Medicine
Defibrillators are used in sports medicine to treat sudden cardiac arrest (SCA) in athletes. AEDs are often located at sports venues to be used in case of an emergency.
Military Use
Defibrillators may be used in military settings to treat soldiers who have suffered SCA or other cardiac emergencies.
Transportation
Defibrillators may be carried on planes, trains, and other forms of transportation to be used in case of an emergency.
Home Use
Some people may have an AED in their home if they or a family member is at high risk of SCA.
Implantable Defibrillators
These devices are surgically implanted in the chest and are used to treat arrhythmias that cannot be controlled with medication. They are typically reserved for people who have had a heart attack or who have other conditions that put them at high risk of SCA.
Standards
Defibrillators are medical devices that are subject to a number of standards and regulations to ensure their safety and effectiveness. In the United States, defibrillators are regulated by the Food and Drug Administration (FDA), which is responsible for regulating the production, sale, and use of medical devices.
Some of the standards and regulations that apply to defibrillators include:
International Electrotechnical Commission (IEC) 60601 Series
This international standard establishes safety and performance requirements for medical electrical equipment and systems. Defibrillators must meet the requirements of IEC 60601-1, which outlines the general requirements for safety and essential performance, as well as any additional requirements specified in other parts of the standard.
ISO 13485
This international standard outlines the requirements for a quality management system specific to the medical device industry. Defibrillator manufacturers must have a quality management system in place that meets the requirements of this standard in order to produce and sell their products.
FDA Regulations
In the United States, defibrillators are subject to a number of FDA regulations, including the Medical Device Amendments of 1976, the Safe Medical Devices Act of 1990, and the Food and Drug Administration Modernization Act of 1997. These regulations establish the criteria for the approval of medical devices, including defibrillators, and outline the requirements for their production, labeling, and marketing.
In addition to these standards and regulations, defibrillators must also meet the requirements of local and national regulatory agencies in the countries where they are sold. This may include additional safety and performance standards, as well as requirements for labeling and advertising.
Overall, the standards and regulations that apply to defibrillators help to ensure that these devices are safe and effective for use in the medical setting and in public places, and that they meet the necessary performance and safety standards. Defibrillators save lives and have to work when they are needed.
Related Information
References