-
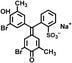
Formation of the bisulfite anion (HSO 3 – , m/z 81) upon collision‐induced dissociation of anions derived from organic sulfonic acids
2-Sulfoacetic acid (16), 3-sulfopropanoic acid (17), 4-sulfobutanoic
acid (18), and 6-sulfohexanoic acid (19) were also synthesized
by the Strecker sulfite alkylation .[29,30]
Bromoacetic acid (50 mg,
0.360 mmol), 3-bromopropanoic acid (50 mg, 0.327 mmol …
-
Asymmetric Nucleophilic Glyoxylation through a Metalated α‐Aminonitrile Derivative in Michael Additions to Nitroalkenes
base[6]
or through b elimination of a diol cyclic sulfite .[7]
In several methods, the concept … as the C2 nucleophile.[10]
Furthermore, several alkylation reactions of lithiated 1,3-dithiane-protected alkyl glyoxylates … our attempts to synthesize
the glyoxylate aminonitrile 2, we first tried an asymmetric Strecker reaction starting with …
-
Alkylethoxyethanesulphonates: Two techniques for improving synthetic conversions
Replacement of sodium sulphite by potassium sulphite increases conversion still further, to quantita-
tive, in the sulphonation … The ethoxylation of long-chain fatty alcohols or the
products of phenol alkylation with detergent range
a … These products were first patented in 1938 by Bruson
(7} who applied the Strecker sulphonation procedure
to …
-
Food chemistry
… maturation 551 -, degradation through lipid peroxidation 217 218(T), 217(A), -, acylation 18 -, alkylation 20 -, analysis 42 … … Sensory relevance, determination 356 -, sorption 399, 399(A) -, -, binding constant 399 -, stability 405 -, extensor -aldehyde 367, 367 … … 934(A), 934(T) -, flavoring agent 931 934(A), 932(T), -, formation of sulfite 935 -, bitter material …
-
Food Chemistry
… reaction with monohydroperoxide 212, 213
–, reaction with pyridoxal phosphate 065
–, reductive alkylation 080(T)
– … … T)
–, temperature of phase transition 006(T)
Strawberry juice, adulteration 858(T) Strecker acid, formation 283 … … Sugar, L-
–, synthesis 878
Sulfhydrylation, reductive 364, 365
Sulfitation, sugar extract 870 Sulfite , antimicrobial action 452 …
-
Organic chemistry
… 1036-Desoxyribo- 1008 Nucleolus 726 Nucleophil to 1049 ribo Nucleobasen 154, 333 156- alkylation 336, Alkinyl anions … … 658 material balance 394 material classes, overview 6 metabolisms 319 strand, delayed 1048 extensor -synthesis 895 bar … … 725 Succinat 592, 594 succinimide 594 Sucrase 817 suffix 43 sulfanilic acid 843 sulfite ablauge 825 sulfite …
-
Encyclopedia of Catalysis 6 Volume Set
In the second
case, a simple 'antifluorescein antibody displayed weak catalysis of sulfite reduc-
tion of a … These reactions include etherifi-
cation, hydrolysis, esterification, alkylation , hydrogenation, isomerization, desul-
furization, amination, aldol condensation, olefin oligomerization … These types of catalysts
have been used for enantioselective Strecker reactions and Mannich-type reac-
tions (144 …
-
March's Advanced Organic Chemistry: Reactions Mechanisms and Structure 6th Edition Complete Document
1448 For a review of asymmetric Strecker syntheses, see Williams, R.M. Synthesis of Optically Active a … … 1173 Carboxylic esters can be formed from the carboxylate anion and a suitable alkylating agent (10-26). Besides zinc, sodium sulfite , hydrazine, sodium sulfide, and other reducing agents have been used.
-
Handbook of Industrial Chemistry and Biotechnology
Debeire P, Priem B, Strecker G, Vignon M 1991) ASAM alkaline sulfite pulping process shows
potential for large-scale application. The resulting “hard” alkylate was a
highly branched chain compound.
-
Glycoscience
… sulfite, which can absorb formalde-
hyde, glycoaldehyde, and glyceraldehyde by adduct formation with the immobilized sulfite anions. … S)-(−)-amino-
2-(methoxymethyl)pyrrolidine] gives hydrazone 129 which is deprotonated and alkylated with
methyl iodide … The aforementioned D-galactose-derived chiral imines 27 were used for the Strecker synthesis
of D-α …
 Chemical Manufacturing and Synthesis Services - (349 companies)Chemical manufacturing and synthesis services produce organic chemicals and inorganic chemicals on a contract, toll, customized, or research and development basis.Chemicals / Chemical ProductsRun / Batch SizeLocation
Chemical Manufacturing and Synthesis Services - (349 companies)Chemical manufacturing and synthesis services produce organic chemicals and inorganic chemicals on a contract, toll, customized, or research and development basis.Chemicals / Chemical ProductsRun / Batch SizeLocation Processing Services - (1809 companies)Chemical and material processing services modify chemicals and materials and offer a wide range of services.
Processing Services - (1809 companies)Chemical and material processing services modify chemicals and materials and offer a wide range of services. Biotechnology and Pharmaceutical Manufacturing Services - (454 companies)Biotechnology and pharmaceutical manufacturing services activate pharmaceutical ingredients, sterilize biomedical components and materials, validate processes or packaging, and formulate drug or medicinal preparations on a contract basis.
Biotechnology and Pharmaceutical Manufacturing Services - (454 companies)Biotechnology and pharmaceutical manufacturing services activate pharmaceutical ingredients, sterilize biomedical components and materials, validate processes or packaging, and formulate drug or medicinal preparations on a contract basis. Water Quality Photometers and Colorimeters - (151 companies)Water quality photometers and colorimeters are ion-specific, computer-interfaced probes that are designed to determine the concentration of a solution from its color intensity.
Water Quality Photometers and Colorimeters - (151 companies)Water quality photometers and colorimeters are ion-specific, computer-interfaced probes that are designed to determine the concentration of a solution from its color intensity. Food Additives - (417 companies)Butylated hydroxyanisole (BHA) - free radical scavenger. Butylated hydroxytoluene (BHT) - free radical scavenger. Citric acid - enzyme inhibitor. Sulfites - enzyme inhibitor. Tertiary butylhydroquinone (TBHQ) - free radical scavenger. Tocopherols...
Food Additives - (417 companies)Butylated hydroxyanisole (BHA) - free radical scavenger. Butylated hydroxytoluene (BHT) - free radical scavenger. Citric acid - enzyme inhibitor. Sulfites - enzyme inhibitor. Tertiary butylhydroquinone (TBHQ) - free radical scavenger. Tocopherols... Indicates content that may require registration and/or purchase.
Indicates content that may require registration and/or purchase.