The industry's most authoritative handbook on flow measurement provides a road map to the field of flow measurement. This best-seller discusses strategies for problem solving and puts the whole array of types of flowmeters at the reader's disposal. The text includes laminar flow elements, critical flowmeters, statistics for measurement, laboratory primary standards, and uncertainty in flow measurement. Emphasis is placed on the importance of accuracy in measurements and ways of ensuring accuracy and avoiding equipment damage through correct forecast of operating conditions, flowmeter selection, installation, calibration, and maintenance. Fundamental considerations such as mixed-phase flow, piping effects, and flow conditioning are examined at length. The problem of attaining a meaningful flow signal through linearization, compensation, and totalization is discussed. Join the thousands of engineers, technicians, managers, and salespeople that have found this reference text an invaluable resource.
The industry's most authoritative handbook on flow measurement provides a road map to the field of flow measurement. This best-seller discusses strategies for problem solving and puts the whole array of types of flowmeters at the reader's disposal. The text includes laminar flow elements, critical flowmeters, statistics for measurement, laboratory primary standards, and uncertainty in flow measurement. Emphasis is placed on the importance of accuracy in measurements and ways of ensuring accuracy and avoiding equipment damage through correct forecast of operating conditions, flowmeter selection, installation, calibration, and maintenance. Fundamental considerations such as mixed-phase flow, piping effects, and flow conditioning are examined at length. The problem of attaining a meaningful flow signal through linearization, compensation, and totalization is discussed. Join the thousands of engineers, technicians, managers, and salespeople that have found this reference text an invaluable resource.
Flow Measurement
Chapter 3 - Physical Properties of Fluids: Gas Compressibility Factor
Unfortunately, the Ideal Gas Law is accurate only at relatively low pressures
and high temperatures. To account for the deviation from the ideal situation,
another factor is included. It is called the Gas Compressibility Factor, or Z-factor.
This correction factor is dependent on pressure and temperature for each gas considered.
The True Gas Law, or the Non-Ideal Gas Law, becomes:
| PV = ZnRT | (3-4) | |
| and density becomes: | ||
 | (3-5) |
| The Z-factor should always be considered when calculating a gas density. It is equal to unity at low pressures and high temperatures but can deviate widely from unity under other conditions. Some gases are not ideal even when at atmospheric pressure and ambient temperature. | The Ideal Gas Law is only a crude approximation. Be sure to include the Z-factor in your gas density calculations. |
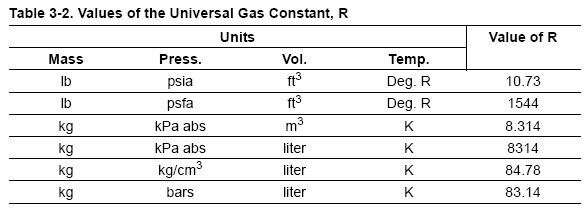
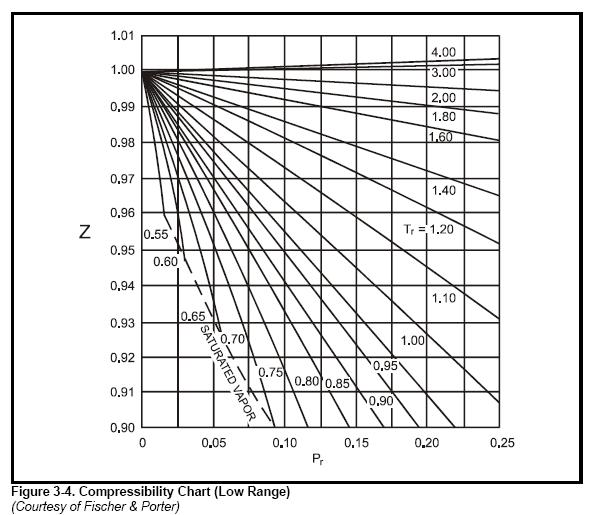
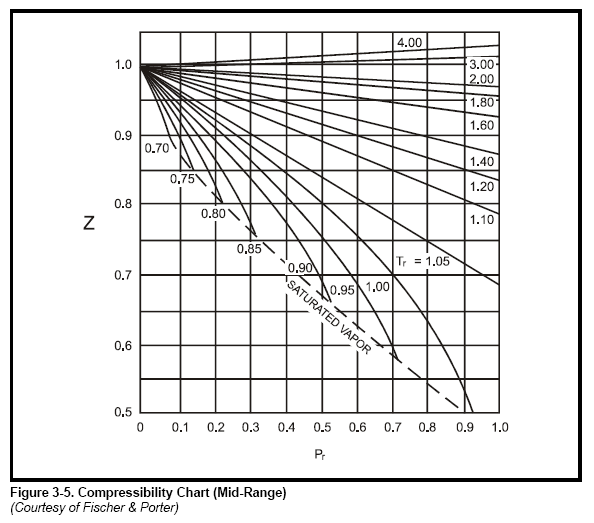
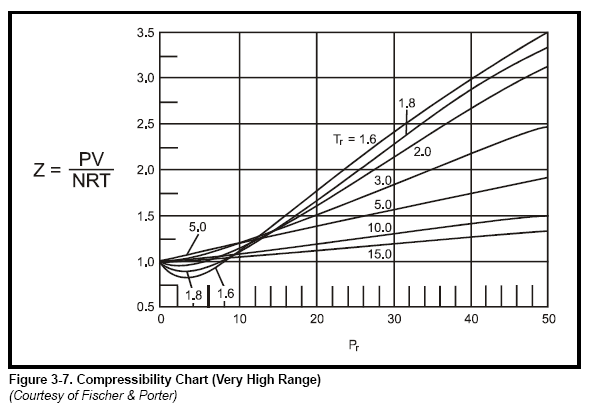
most common method to find Z is to use the normalized compressibility charts.
These charts offer a remarkable correlation of Z-factor with the pressure and temperature
of gases normalized with respect to their critical values. Critical pressure
and critical temperature are the conditions above which liquid cannot exist. Critical
values for a number of gases are listed in Table 3-3. Graphs in Figure 3-4, Figure
3-5, Figure 3-6, and Figure 3-7 give Z-factors from values of reduced pressure
and temperature. Accuracy is better than 2% for most gases. In some cases the
critical values in Table 3-3 have been slightly modified so that the Z-charts are
more accurate.
The normalized or "reduced" pressure and temperature values are calculated as:
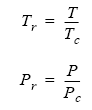 | (3-6) | |
| (3-7) |
| where: | |||
| Tr | = | reduced temperature | |
| Pr | = | reduced pressure | |
| T | = | absolute temperature | |
| P | = | absolute pressure | |
| Tc | = | critical temperature | |
| Pc | = | critical pressure |
T and Tc and also P and Pc must be in the same units.
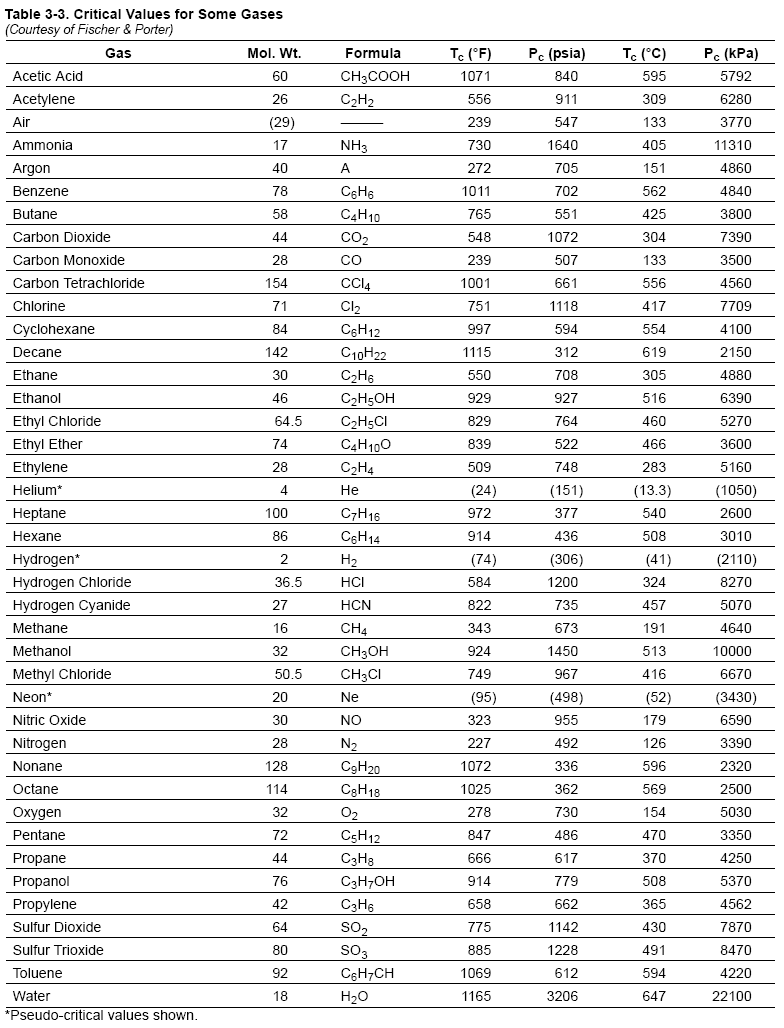

| When accurate tables or graphs of density at different operating conditions exist, it is not necessary to use the Z-charts and the Ideal Gas Law. For example, very accurate values for specific volumes of steam are presented in tables of the thermodynamic properties of steam. In the case of natural gas and other hydrocarbon mixtures high in methane, the American Gas Association has published Z-factors based on five criteria: pressure, temperature, specific gravity, % carbon dioxide, and % nitrogen. This report is called AGA Report No. 3. More recently, AGA has published a very comprehensive method (AGA Report No.8) to solve for the Z-factor of hydrocarbon-based gases with as many as 20 different components in varying proportions. | Data for the density of air are rather scarce. Fortunately, air acts very nearly as an ideal gas if operating below 1400 kPa abs and above 0°C (204 psia and 32°F). Use the Ideal Gas Law with an approximate molecular weight of 29 for air. |
© 2001 ISA
Featured Products
 TABLE OF CONTENTS
TABLE OF CONTENTS