Engineering Chemistry

| 1. | What is meant by available chlorine ? |
|
| 2. | What do you mean by iodometric titration? |
|
| 3. | Write the formula of bleaching powder. |
|
| 4. | Write the chemical reactions taking place in this experiment. |
|
| 5. | What is the other name of bleaching powder? |
|
| 6. | What is the approximate percentage of available chlorine in fresh commercial bleaching powder? |
|
| 7. | Name the indicator used in the determination of available chlorine in the sample of bleaching powder. |
|
| 8. | Give examples of chemical sanitizers. |
|
| 9. | Why is only freshly prepared starch used as indicator? |
|
| 10. | What is the color change at the end point? |
|
| 11. | Why does the blue color appear on the addition of starch? |
|
| 12. | What are the main constituents of bleaching powder? |
|
| 13. | Give two applications of bleaching powder. |
|
| 14. | Why should the starch indicator be added near the end point? |
|
| 15. | Why does the blue color disappear at the end point? |
|
| 16. | Why should iodometric titrations not be performed at high temperature? |
|
| 17. | Why is an excess of KI always used? |
|
Answers
| 1. | Available chlorine is the amount of chlorine liberated by the action of dilute mineral acids on bleaching powder. |
| 2. | The titration in which liberated iodine is titrated against standard solution of sodium thiosulphate is called iodometric titration. |
| 3. | CaOCl 2. |
| 4. |
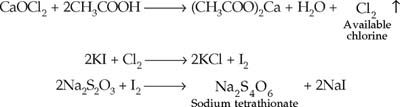 |
| 5. | Chloride of lime or Bleach. |
| 6. | 36 38%. |
| 7. | Freshly prepared starch solution is used as indicator. |
| 8. | Sodium hypochlorite and bleaching powder are widely used for sanitization. |
| 9. | Because an... |
